Advanced Point of Care Diagnostics: Interview with PDRC's Dr. Daniel Perez
This interview originally appeared in Update, October 2025 - No. 2, by Royal GD.
When it comes to poultry production, the threat of avian influenza means we need to improve our diagnostic technologies so we can detect and respond to the disease quickly. Because, how do you get a grip on bird flu? And how can you use the available diagnostics and improve management to prevent outbreaks and reduce the potential threat of zoonosis?
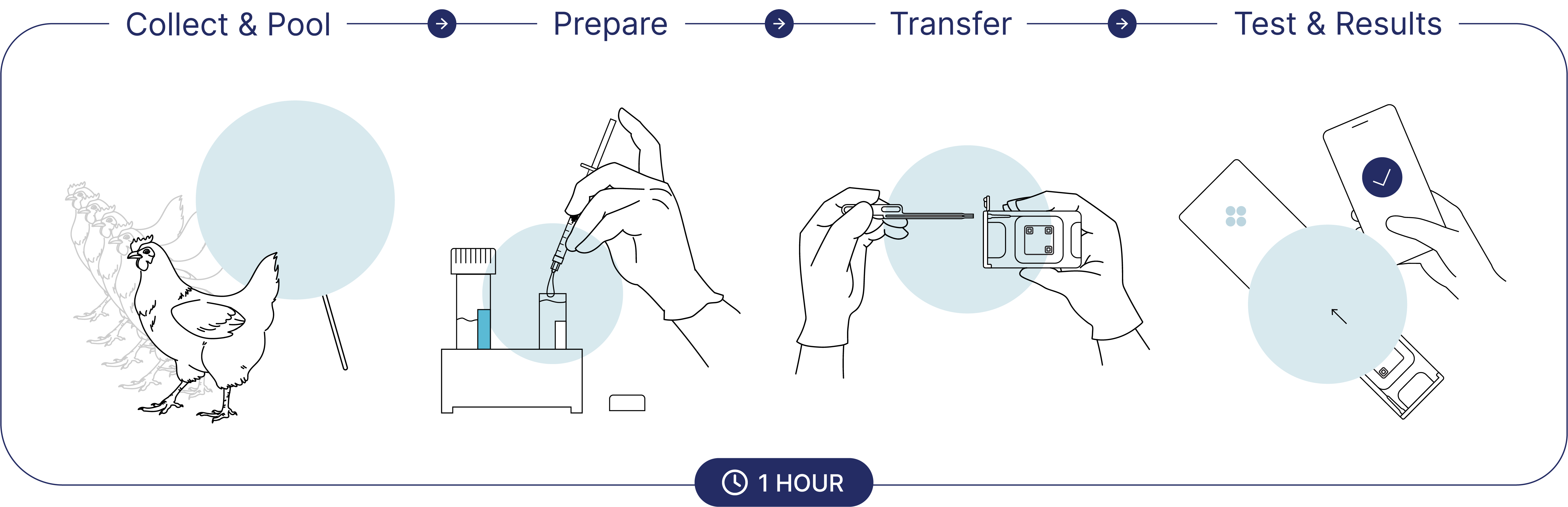
Alveo Technologies, Inc. and Royal GD decided to join forces and developed an on-site poultry avian influenza test: Alveo Sense™. This test gives you accurate, laboratory-grade results whenever you need them, using a cloud-connected, data-driven platform.
This technology is currently being used across the European Union, the Middle East, and Southeast Asia and parts of Africa. The Poultry Diagnostics Research Center (PDRC) conducted a two part study to see how well the Alveo Sense™ test could spot high and low pathogenic H5N1 viruses in chickens and ducks. In the first phase, ducks were monitored over five days with daily collection of oropharyngeal and cloacal swabs. The samples were analysed to determine viral load and evaluate the sensitivity of the test. The second phase was all about testing out the effects on chickens and ducks, and that took six days.
The PDRC study has shown how well the Alveo Sense™ test can detect the H5N1 virus in chickens and ducks during the early stages of infection. PDRC is a renowned global player in the College of Veterinary Medicine at the University of Georgia. It’s about giving poultry producers the support they need, whether it’s through teaching, research, helping out with diagnostics, or offering expert advice.
High-resented accuracy and sensitivity
Initial testing revealed an impressive >99% accuracy in detecting Type A and H5 targets across all tests, including from pooled cloacal swabs in ducks, which are considered the most complex sample type. The early time-to-result values underscored the platform’s strong analytical sensitivity, confirming its suitability for detecting viruses even at low levels. The findings support the utility of the Alveo Sense™ test for field-based surveillance efforts, enabling the feasibility of sample pooling strategies.
Dr. Daniel Perez from PDRC presented the study findings at the 15th International Seminar on Poultry Pathology and Production.
What are the results or relevant findings?

“In the first study, we used Alveo’s kit to monitor HPAIV in cloacal (CL) swabs from ducks challenged with 10^4 TCID50 of an H5N1 strain. Daily sampling over five days demonstrated that even when only one duck was positive, the kit generated a detectable signal. This sensitivity highlights the potential of the platform for early detection in real-world settings. The study was concluded on day 5 when the ducks reached humane endpoints, but the data clearly demonstrated the kit’s ability to detect low-level positives within pooled samples.
In the second study, we challenged both ducks and chickens with either 10^6 or 10^4 TCID of a low-pathogenic H5N1 strain, with cloacal (CL) and oropharyngeal (OP) samples collected from 5 birds daily, across six days. OP pools from chickens consistently produced type A and H5 signals on days 1, 3, and 5, in line with expected virus shedding dynamics.
“Daily sampling over five days demonstrated that even when only one duck was positive, the kit generated a detectable signal.”
Notably, on day 5, the Alveo Sense kit provided a positive signal even when virus levels were below the limit of detection, underscoring the system’s sensitivity. While chicken CL swabs returned inconsistent results, this is likely attributable to sample type or workflow deviation rather than test performance. In ducks, OP samples reliably detected type A and H5 signals on days 2 and 4, and CL samples yielded positives on day 4, demonstrating strong agreement with viral dynamics.”
Can you elaborate on the specific challenges that you encountered while testing the Alveo Sense™ Poultry Avian Influenza test, and how these were addressed?
“Testing in LPAIV-H5N1 challenged ducks showed results consis-tent with viral load measurements, and even partial detections provided actionable information. At 2 DPI, OP swabs were positive for H5 and CL swabs were positive for Type A, demonstrating that the test could reliably flag samples that would warrant confirmatory testing in the field.
In LPAIV-H5N1 challenged chickens, OP pools performed as expected, confirming that the test could track infection dynamics in this specie, while CL pools produced some irregular determine viral load (log10 TCID50/mL) and to assess the sensitivity of the Alveo Sense Poultry Avian Influenza Test Type A H5 H9.
PDRC determined viral titers by infecting Mammalian Madin-Darby Canine Kidney (MDCK) cells with serial dilutions of each individual swab1. TCID50 values were calculated using the Reed and Muench method and expressed as log10 TCID50/mL.
PDRC followed the intended user workflow of the Alveo Sense test in running the cartridges. The Alveo Sense test was run three times during the study, using pooled cloacal swabs from all five birds on days 1, 3, and 5 post-infection. Results via the mobile application were reported qualitatively, in line with the current design of the Alveo Sense platform. The raw data from all three runs were also extracted and analyzed for further evaluation.”

Are there any comparable diagnostic tools currently available on the market? If so, how does the Alveo Sense™ test compare to these existing methods in terms of sensitivity, specificity, and ease of use?
“While I’m not familiar with the full spectrum of available tests, our limited comparison of the Alveo Sense kit and a standard commercial antigen capture test showed a clear performance advantage for the Alveo Sense test. This is primarily because this test uses nucleic acid amplification, a more sensitive method than the antigen-capture test’s reliance on viral antigen concentration. Crucially, I see both types of assays as valuable and complementary tools for outbreak management, allowing for diverse strategies to facilitate rapid intervention and effectively curtail influenza spread.”
You recently presented at the FAO meeting. How did that opportunity arise, what was presented and what was the feedback?
“Following the initial presentation at UGA in August, I’ve continued to share findings from our initial stages of the evaluation of Alveo Sense at congresses in Pennsylvania and recently the FAO meeting in Brazil. It is my belief that tools like the Alveo Sense point of need test have the potential to improve when and how we respond to outbreaks. Ultimately allowing for stronger biosecurity efforts to minimize the impact both on site and at surrounding facilities. The information was well received in Brazil with those in attendance understanding the value of a tool like this.”
What implications do your study findings have for the future of avian influenza surveillance and control strategies on a global scale?
“The FAO meeting ended with attendees collaborating to establish a list of six recommendations for government entities to use to battle avian influenza. That included an emphasis on the use of data technology. There is a strong need to utilize data technology for diagnostic advancements or centralized real-time data management. A capability that this test can support via real-time Geotagging.
“Preventing and controlling avian influenza in poultry requires reliable surveillance systems capable of promptly tracking vaccine breakthroughs in the field.”
Further, these studies demonstrated that molecular testing with Alveo Sense Poultry Avian Influenza test produces highly sensitive results at virus shedding levels that are clinically relevant. These findings support the performance of the test in the field to enable rapid decision making, timely action and better outcomes. Now more than ever, this type of solution is essential as the bird flu catastrophe continues to worsen.”
Is there anything else you’d like to share about this project or anything else that PDRC is currently working on to com-bat Avian Influenza?
“Integrating the Alveo Sense test into our avian influenza research at the PDRC – including studies on interspecies trans-mission, pathogenesis, and vaccine development – is a significant step forward. The assay’s excellent sensitivity and specificity are critical for accurately assessing protection against virus shedding in our vaccination and challenge studies. Ultimately, preventing and controlling avian influenza in poultry requires reliable surveillance systems capable of promptly tracking vac-cine breakthroughs in the field. I am confident that Alveo’s technology will be an invaluable component of this necessary surveillance infrastructure.”

.svg)





